
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- COVID-19
- Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea
- Sung-Youn Chun, Dong Wook Kim, Sang Ah Lee, Su Jung Lee, Jung Hyun Chang, Yoon Jung Choi, Seong Woo Kim, Sun Ok Song
- Diabetes Metab J. 2020;44(6):897-907. Published online December 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0199
- 7,623 View
- 145 Download
- 8 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study aimed to determine the infection risk of coronavirus disease 2019 (COVID-19) in patients with diabetes (according to treatment method).
Methods
Claimed subjects to the Korean National Health Insurance claims database diagnosed with COVID-19 were included. Ten thousand sixty-nine patients with COVID-19 between January 28 and April 5, 2020, were included. Stratified random sampling of 1:5 was used to select the control group of COVID-19 patients. In total 50,587 subjects were selected as the control group. After deleting the missing values, 60,656 subjects were included.
Results
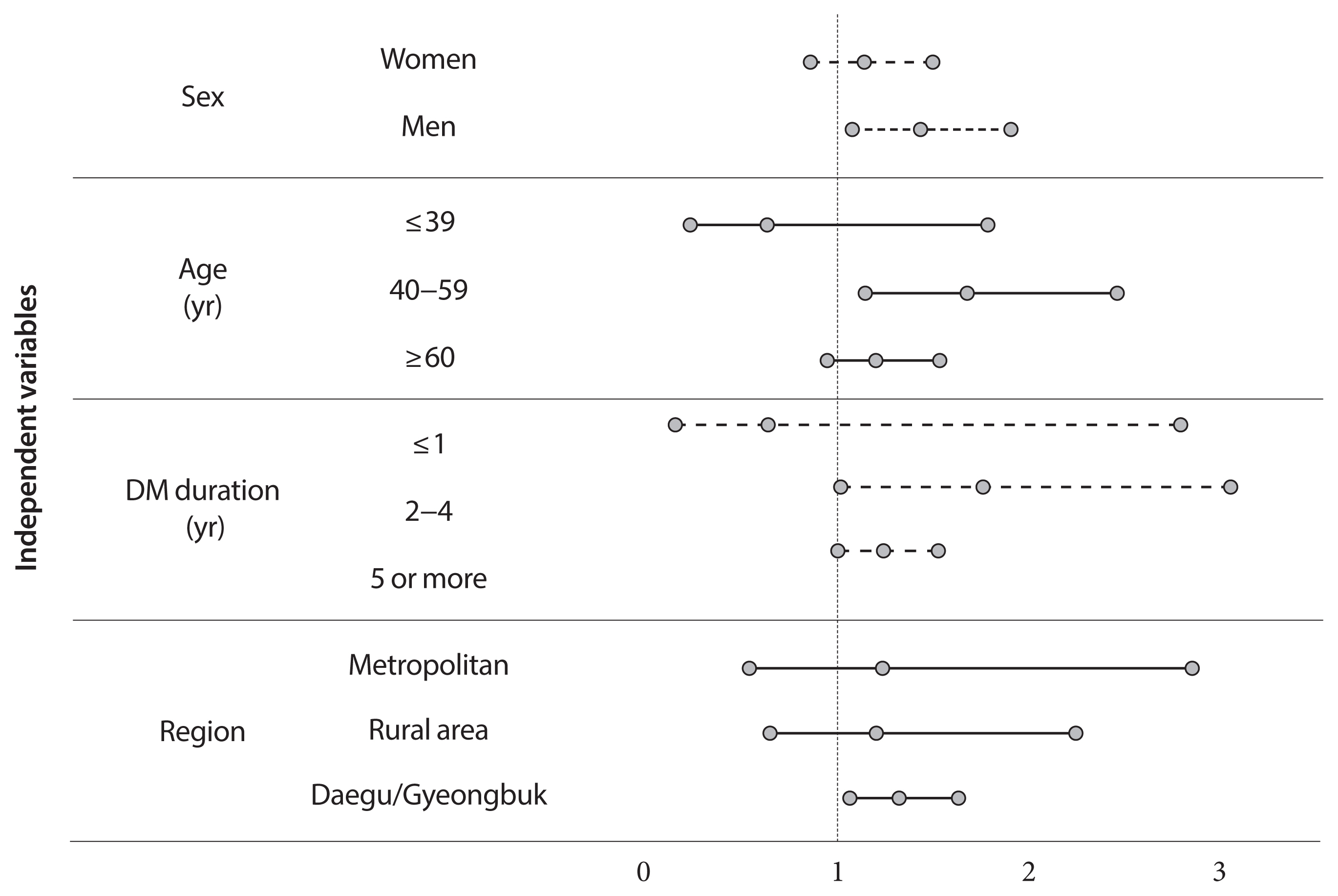
Adjusted odds ratio (OR) indicated that diabetic insulin users had a higher risk of COVID-19 than subjects without diabetes (OR, 1.25; 95% confidence interval [CI], 1.03 to 1.53; P=0.0278). In the subgroup analysis, infection risk was higher among diabetes male insulin users (OR, 1.42; 95% CI, 1.07 to 1.89), those between 40 and 59 years (OR, 1.66; 95% CI, 1.13 to 2.44). The infection risk was higher in diabetic insulin users with 2 to 4 years of morbidity (OR, 1.744; 95% CI, 1.003 to 3.044).
Conclusion
Some diabetic patients with certain conditions would be associated with a higher risk of acquiring COVID-19, highlighting their need for special attention. Efforts are warranted to ensure that diabetic patients have minimal exposure to the virus. It is important to establish proactive care and screening tests for diabetic patients suspected with COVID-19 for timely disease diagnosis and management. -
Citations
Citations to this article as recorded by- Risk factors for SARS-CoV-2 infection during the early stages of the COVID-19 pandemic: a systematic literature review
Matthew Harris, John Hart, Oashe Bhattacharya, Fiona M. Russell
Frontiers in Public Health.2023;[Epub] CrossRef - Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study
Brenda Eskenazi, Stephen Rauch, Enrico Iurlaro, Robert B. Gunier, Albertina Rego, Michael G. Gravett, Paolo Ivo Cavoretto, Philippe Deruelle, Perla K. García-May, Mohak Mhatre, Mustapha Ado Usman, Mohamed Elbahnasawy, Saturday Etuk, Raffaele Napolitano, S
American Journal of Obstetrics and Gynecology.2022; 227(1): 74.e1. CrossRef - The Role of Diabetes and Hyperglycemia on COVID-19 Infection Course—A Narrative Review
Evangelia Tzeravini, Eleftherios Stratigakos, Chris Siafarikas, Anastasios Tentolouris, Nikolaos Tentolouris
Frontiers in Clinical Diabetes and Healthcare.2022;[Epub] CrossRef - COVID-19 and Gestational Diabetes: The Role of Nutrition and Pharmacological Intervention in Preventing Adverse Outcomes
Ruben Ramirez Zegarra, Andrea Dall’Asta, Alberto Revelli, Tullio Ghi
Nutrients.2022; 14(17): 3562. CrossRef - A Comprehensive Analysis of Chinese, Japanese, Korean, US-PIMA Indian, and Trinidadian Screening Scores for Diabetes Risk Assessment and Prediction
Norma Latif Fitriyani, Muhammad Syafrudin, Siti Maghfirotul Ulyah, Ganjar Alfian, Syifa Latif Qolbiyani, Muhammad Anshari
Mathematics.2022; 10(21): 4027. CrossRef - The World-Wide Adaptations of Diabetic Management in the Face of COVID-19 and Socioeconomic Disparities: A Scoping Review
Jaafar Abou-Ghaida, Annalia Foster, Sarah Klein, Massah Bassie, Khloe Gu, Chloe Hille, Cody Brown, Michael Daniel, Caitlin Drakeley, Alek Jahnke, Abrar Karim, Omar Altabbakh, Luzan Phillpotts
Cureus.2022;[Epub] CrossRef - Dissection of non-pharmaceutical interventions implemented by Iran, South Korea, and Turkey in the fight against COVID-19 pandemic
Mohammad Keykhaei, Sogol Koolaji, Esmaeil Mohammadi, Reyhaneh Kalantar, Sahar Saeedi Moghaddam, Arya Aminorroaya, Shaghayegh Zokaei, Sina Azadnajafabad, Negar Rezaei, Erfan Ghasemi, Nazila Rezaei, Rosa Haghshenas, Yosef Farzi, Sina Rashedi, Bagher Larijan
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1919. CrossRef
- Risk factors for SARS-CoV-2 infection during the early stages of the COVID-19 pandemic: a systematic literature review
- Intracerebroventricular Injection of Metformin Induces Anorexia in Rats
- Chang Koo Lee, Yoon Jung Choi, So Young Park, Jong Yeon Kim, Kyu Chang Won, Yong Woon Kim
- Diabetes Metab J. 2012;36(4):293-299. Published online August 20, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.4.293
- 4,455 View
- 42 Download
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Metformin, an oral biguanide insulin-sensitizing agent, is well known to decrease appetite. Although there is evidence that metformin could affect the brain directly, the exact mechanism is not yet known.
Methods To evaluate whether metformin induces anorexia via the hypothalamus, various concentrations of metformin were injected into the lateral ventricle of rats through a chronically implanted catheter and food intake was measured for 24 hours. The hypothalamic neuropeptides associated with regulation of food intake were also analyzed following 1 hour of intracerebroventricular (ICV) injections of metformin.
Results An ICV injection of metformin decreased food intake in a dose-dependent manner in unrestrained conscious rats. Hypothalamic phosphorylated AMP-activated protein kinase (pAMPK) increased by 3 µg with metformin treatment, but there was no further increase in pAMPK with increases in metformin dosage. The hypothalamic phosphorylated signal transducer and activator of transcription 3 (pSTAT3) increased by 3 µg with metformin treatment, but, there was no further increase in pSTAT3 level following increases of metformin dosage. Hypothalamic proopiomelanocortin was elevated with metformin treatment, while neuropeptide Y was not significantly changed.
Conclusion Our results suggest that metformin induces anorexia via direct action in the hypothalamus and the increase in pSTAT3, at least in part, is involved in the process. However, hypothalamic pAMPK appears not to contribute to metformin-induced appetite reduction in normal rats. Further studies exploring new pathways connecting metformin and feeding regulation are needed.
-
Citations
Citations to this article as recorded by- Metabolic and Metabolomic Effects of Metformin in Murine Model of Pulmonary Adenoma Formation
Andrew C. Elton, Vannesa Cedarstrom, Arman Quraishi, Beverly Wuertz, Kevin Murray, Todd W. Markowski, Donna Seabloom, Frank G. Ondrey
Nutrition and Cancer.2023; 75(3): 1014. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Journal of Evolutionary Biochemistry and Physiology.2023; 59(5): 1810. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Российский физиологический журнал им И М Сеченова.2023; 109(10): 1414. CrossRef - Metformin in nucleus accumbens core reduces cue‐induced cocaine seeking in male and female rats
Amy Chan, Alexis Willard, Sarah Mulloy, Noor Ibrahim, Allegra Sciaccotta, Mark Schonfeld, Sade M. Spencer
Addiction Biology.2022;[Epub] CrossRef - Knockdown of Endogenous Nucb2/Nesfatin-1 in the PVN Leads to
Obese-Like Phenotype and Abolishes the Metformin- and Stress-Induced Thermogenic
Response in Rats
Daniel Stephan, Natalie Taege, Riccardo Dore, Julica Folberth, Olaf Jöhren, Markus Schwaninger, Hendrik Lehnert, Carla Schulz
Hormone and Metabolic Research.2022; 54(11): 768. CrossRef - Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight
Vitor Ferreira, Cintia Folgueira, Maria Guillén, Pablo Zubiaur, Marcos Navares, Assel Sarsenbayeva, Pilar López-Larrubia, Jan W. Eriksson, Maria J. Pereira, Francisco Abad-Santos, Guadalupe Sabio, Patricia Rada, Ángela M. Valverde
Metabolism.2022; 137: 155335. CrossRef - Metformin acts on the gut-brain axis to ameliorate antipsychotic-induced metabolic dysfunction
Xiaorong Wang, Huimin Huang, Yiyi Zhu, Shaoli Li, Peifen Zhang, Jiajun Jiang, Caixi Xi, Lingling Wu, Xingle Gao, Yaoyang Fu, Danhua Zhang, Yiqing Chen, Shaohua Hu, Jianbo Lai
BioScience Trends.2021; 15(5): 321. CrossRef - Therapeutic effect of treatment with metformin and/or 4-hydroxychalcone in male Wistar rats with nonalcoholic fatty liver disease
Selene de Jesús Acosta-Cota, Elsa Maribel Aguilar-Medina, Rosalío Ramos-Payán, José Guadalupe Rendón Maldonado, José Geovanni Romero-Quintana, Julio Montes-Avila, Juan I. Sarmiento-Sánchez, Carolina Gabriela Plazas-Guerrero, Marcela J. Vergara-Jiménez, Ar
European Journal of Pharmacology.2019; 863: 172699. CrossRef - The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect
Kira Derkach, Irina Zakharova, Inna Zorina, Andrey Bakhtyukov, Irina Romanova, Liubov Bayunova, Alexander Shpakov, Guillermo López Lluch
PLOS ONE.2019; 14(3): e0213779. CrossRef - Effect of Metformin on Antipsychotic-Induced Metabolic Dysfunction: The Potential Role of Gut-Brain Axis
Chao Luo, Xu Wang, Hanxue Huang, Xiaoyuan Mao, Honghao Zhou, Zhaoqian Liu
Frontiers in Pharmacology.2019;[Epub] CrossRef - Metformin alters signaling induced crosstalk and homeostasis in the carcinogenesis paradigm “Epistemology of the origin of cancer”
Björn L.D.M. Brücher, Ijaz S. Jamall, Obul R. Bandapalli
4open.2019; 2: 12. CrossRef - Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high‐fat‐fed rats
Rosana F. Dantas‐Ferreira, Helene Raingard, Stephanie Dumont, Carole Schuster‐Klein, Beatrice Guardiola‐Lemaitre, Paul Pevet, Etienne Challet
Endocrinology, Diabetes & Metabolism.2018;[Epub] CrossRef -
Molecular Mechanisms of the Effects of Metformin on the Functional Activity of Brain Neurons
A. O. Shpakov, K. V. Derkach
Neuroscience and Behavioral Physiology.2018; 48(8): 969. CrossRef - Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: in vitro and in vivo studies
Ali Taghizadehghalehjoughi, Ahmet Hacimuftuoglu, Meltem Cetin, Afife Busra Ugur, Bianca Galateanu, Yaroslav Mezhuev, Ufuk Okkay, Numan Taspinar, Mehmet Taspinar, Abdullah Uyanik, Betul Gundogdu, Maryam Mohammadzadeh, Kemal Alp Nalci, Polychronis Stivaktak
Nanomedicine.2018; 13(13): 1595. CrossRef - Effect of Betahistine and Metformin on Antipsychotic-Induced Weight Gain: An Analysis of Two Clinical Trials
Dongyu Kang, Zhihui Jing, Ranran Li, Gangrui Hei, Tiannan Shao, Li Li, Mengxi Sun, Ye Yang, Ying Wang, Xiaoyi Wang, Yujun Long, Xiansheng Huang, Renrong Wu
Frontiers in Psychiatry.2018;[Epub] CrossRef - Metformin: not only per os
Lev M. Berstein
Expert Review of Endocrinology & Metabolism.2018; 13(2): 63. CrossRef - МЕТАБОЛИЧЕСКИЕ ПОКАЗАТЕЛИ И ФУНКЦИОНАЛЬНОЕ СОСТОЯНИЕ СИГНАЛЬНЫХ СИСТЕМ ГИПОТАЛАМУСА И ВЛИЯНИЕ НА НИХ МЕТФОРМИНА У МЫШЕЙ С МУТАЦИЕЙ AY/A, ГЕНЕТИЧЕСКИ ПРЕДРАСПОЛОЖЕННЫХ К ОЖИРЕНИЮ, "Доклады Академии наук"
К.В. Деркач, И.О. Захарова, И.В. Романова, И. И. Зорина, А.Л. Михрина, А.О. Шпаков
Доклады Академии Наук.2017; (4): 488. CrossRef - Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats
Ichiro Tokubuchi, Yuji Tajiri, Shimpei Iwata, Kento Hara, Nobuhiko Wada, Toshihiko Hashinaga, Hitomi Nakayama, Hiroharu Mifune, Kentaro Yamada, M. Faadiel Essop
PLOS ONE.2017; 12(2): e0171293. CrossRef - Metabolic parameters and functional state of hypothalamic signaling systems in AY/a mice with genetic predisposition to obesity and the effect of metformin
K. V. Derkach, I. O. Zakharova, I. V. Romanova, I. I. Zorina, A. L. Mikhrina, A. O. Shpakov
Doklady Biochemistry and Biophysics.2017; 477(1): 377. CrossRef - Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice
Joanne S. Allard, Evelyn J. Perez, Koji Fukui, Priscilla Carpenter, Donald K. Ingram, Rafael de Cabo
Behavioural Brain Research.2016; 301: 1. CrossRef - Intracerebroventricular Metformin Decreases Body Weight But Has Pro-oxidant Effects and Decreases Survival
Luis Valmor Portela, Jussania Gnoatto, Andressa Wigner Brochier, Clarissa Branco Haas, Adriano Martimbianco de Assis, Afonso Kopczynski de Carvalho, Gisele Hansel, Eduardo Rigon Zimmer, Jean Pierre Oses, Alexandre Pastoris Muller
Neurochemical Research.2015; 40(3): 514. CrossRef - Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway
Young Mi Song, Yong-ho Lee, Ji-Won Kim, Dong-Sik Ham, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2015; 11(1): 46. CrossRef - Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: Interference of AMPK/PGC-1α pathway
Ghorbangol Ashabi, Fariba Khodagholi, Leila Khalaj, Mahdi Goudarzvand, Masoumeh Nasiri
Metabolic Brain Disease.2014; 29(1): 47. CrossRef - Acute oral metformin enhances satiation and activates brainstem nesfatinergic neurons
Thaïs Rouquet, Pierre Clément, Stéphanie Gaigé, Catherine Tardivel, Julien Roux, Michel Dallaporta, Bruno Bariohay, Jean-Denis Troadec, Bruno Lebrun
Obesity.2014; : n/a. CrossRef - Metformin—mode of action and clinical implications for diabetes and cancer
Ida Pernicova, Márta Korbonits
Nature Reviews Endocrinology.2014; 10(3): 143. CrossRef - Effects of metformin on weight loss
Steven K. Malin, Sangeeta R. Kashyap
Current Opinion in Endocrinology, Diabetes & Obesity.2014; 21(5): 323. CrossRef - The effect of ghrelin on MK-801 induced memory impairment in rats
Fatemeh Goshadrou, Mojtaba Kermani, Abdolaziz Ronaghi, Samad Sajjadi
Peptides.2013; 44: 60. CrossRef
- Metabolic and Metabolomic Effects of Metformin in Murine Model of Pulmonary Adenoma Formation

 KDA
KDA
 First
First Prev
Prev





